Patient Design Studio
Transforming the Clinical Trial Experience Through Patient-Centered Design
The Patient Insights Program was a cross-functional effort to bring patient voices directly into product development. In my role as Lead Designer for Medidata Patient Cloud, I guided the design activities, supported synthesis of insights into practical tools, and collaborated with product and engineers to integrate patient perspectives into roadmap decisions.
The Challenge
Clinical trials are essential for advancing medicine, yet for many patients the experience feels confusing, overwhelming, and impersonal. Participants often described feeling like passive subjects, even though their engagement is critical to a study’s success. Our goal was to understand the patient perspective in depth and translate it into solutions that felt intuitive, compassionate, and human-centered.

Patient Voices
When we asked patients to share their experiences, their words revealed how often they felt unseen, unsupported, or rushed through important decisions.
“My time isn’t valued and it’s difficult to commit to everything without greatly inconveniencing myself and my family.”
Participation placed impossible demands on daily life.
“I don’t have support or feedback after consenting and I feel isolated.”
Patients felt abandoned once the study began.
“I often don’t understand everything I’m signing up for and I don’t know how to get clarity when I’m confused.”
Critical decisions were made without true understanding.
“I don’t have the option to learn at my own pace or style and it’s frustrating.”
Patients had no control over how they learned.
Opportunity & Approach
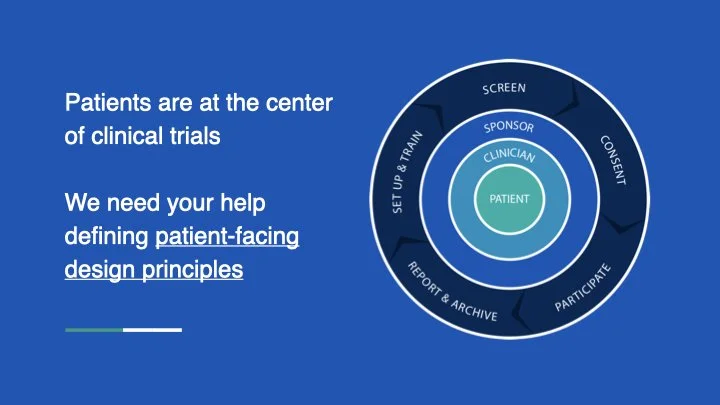
These perspectives revealed systemic gaps in communication, support, and accessibility. To address them, my team established Patient Design Studios, a series of design thinking workshops that brought together patients, clinicians, designers, product managers, and engineers.
These sessions allowed us to:
Align on project goals and priorities
Map the patient journey, with emotional highs and lows
Sketch innovative ideas to reimagine trial participation
Prioritize features of the new experience
Similar workshops and collaborative sessions were held at the start of subsequent sprints, serving as checkpoints for alignment and shared problem solving across disciplines.
Design Activities
Building on patient insights, we used a mix of research and collaborative methods to translate experiences into actionable steps.
Patient Interviews: I helped conduct interviews with twenty-one participants across diverse study types and conditions. Their stories revealed emotional pain points, unmet needs, and behavioral patterns that shaped our priorities.
Journey Mapping: We mapped trial touchpoints with patients and stakeholders, capturing emotional highs and lows and gaps in support. These maps became a shared reference for design, product, and engineering.
Co-Creation Workshops: I facilitated sessions where patients worked alongside our teams to generate ideas for key moments such as consent and eligibility screening.
Continuous Validation: We partnered with patients and subject-matter experts across the full lifecycle, including QA and final design reviews, to ensure the experience addressed real needs and reduced friction.
Design Principles: Together we translated research into guiding product principles centered on trust, clarity, and empathy, which informed day-to-day design decisions.
Patient Journey
The final journey map, built from research and collaborative workshops, captured the patient experience across every stage of a clinical trial. It revealed emotional highs and lows, gaps in communication, and opportunities for better support, and became a critical alignment tool for guiding product development.
Key Insights
Clinical trials often felt transactional during moments that were deeply emotional
Patients lacked sufficient resources and time to make informed decisions
Communication between sites and patients was inconsistent and fragmented
Many participants described feelings of isolation and disconnection: “It feels like there is no one else out there.”
Information was overwhelming, with multiple sources providing conflicting details
Consent and onboarding were rushed, leaving little time for understanding and clarity
Patient Cloud Solutions
The Patient Insights Program, including the design studios, interviews, journey mapping, and feedback sessions, directly shaped the design of Medidata’s Patient Cloud suite. The insights grounded product design in real patient needs, shaping solutions that resolved the most critical barriers to participation.
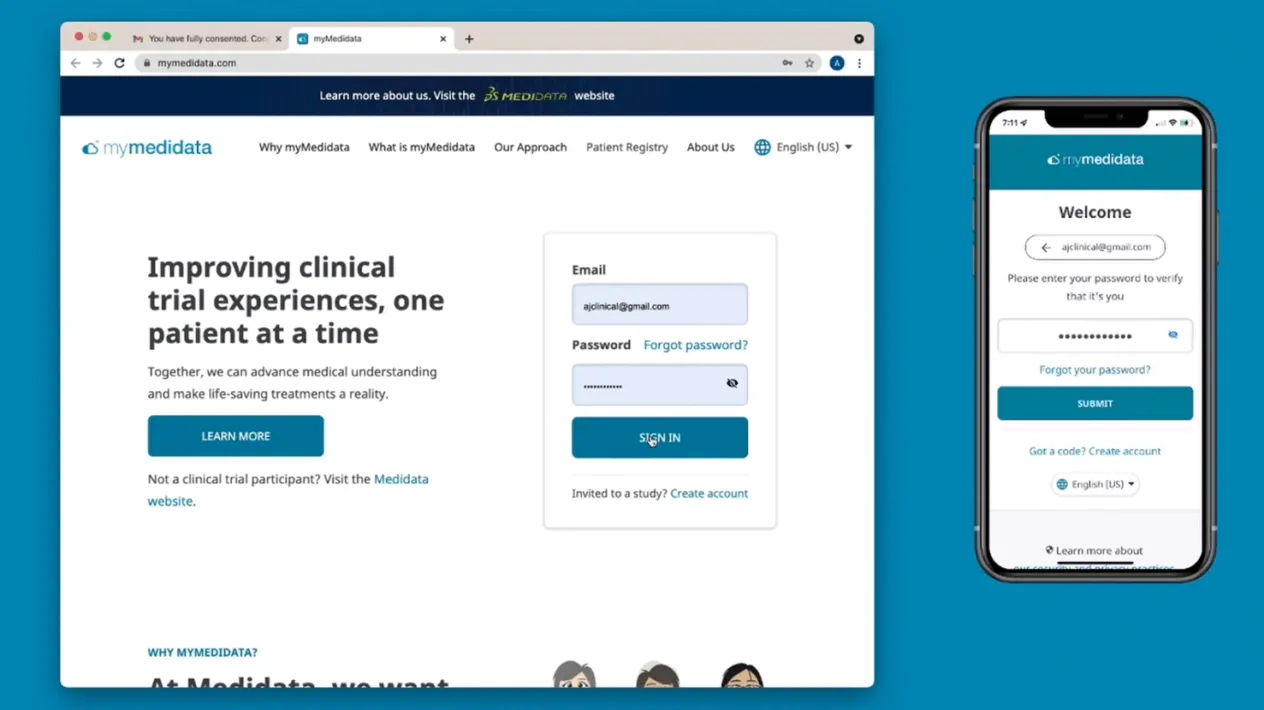
myMedidata
A portal that consolidates study tasks, forms, and video visits into a single experience, reducing fragmentation and information overload.
eConsent
An electronic consent tool that allows patients to review materials at their own pace, flag unclear sections, and return to them with a clinician.
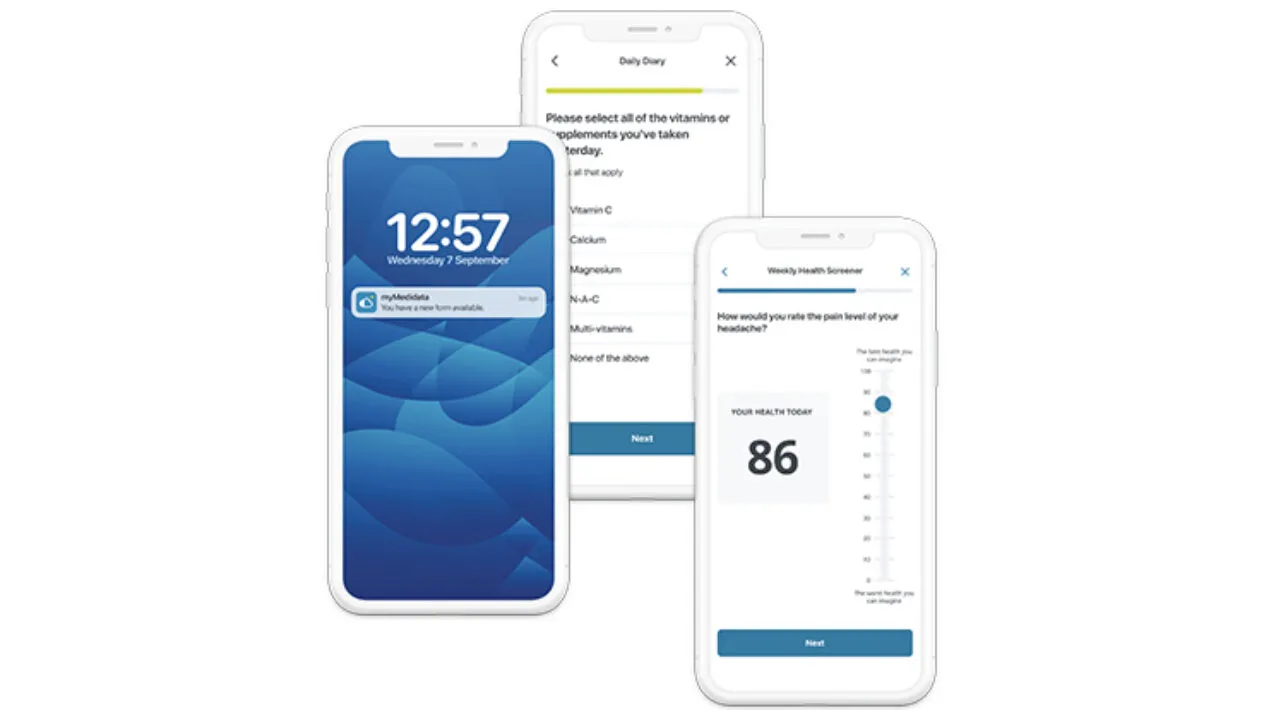
eCOA
A mobile app for completing study assessments remotely, easing daily demands and improving accessibility.
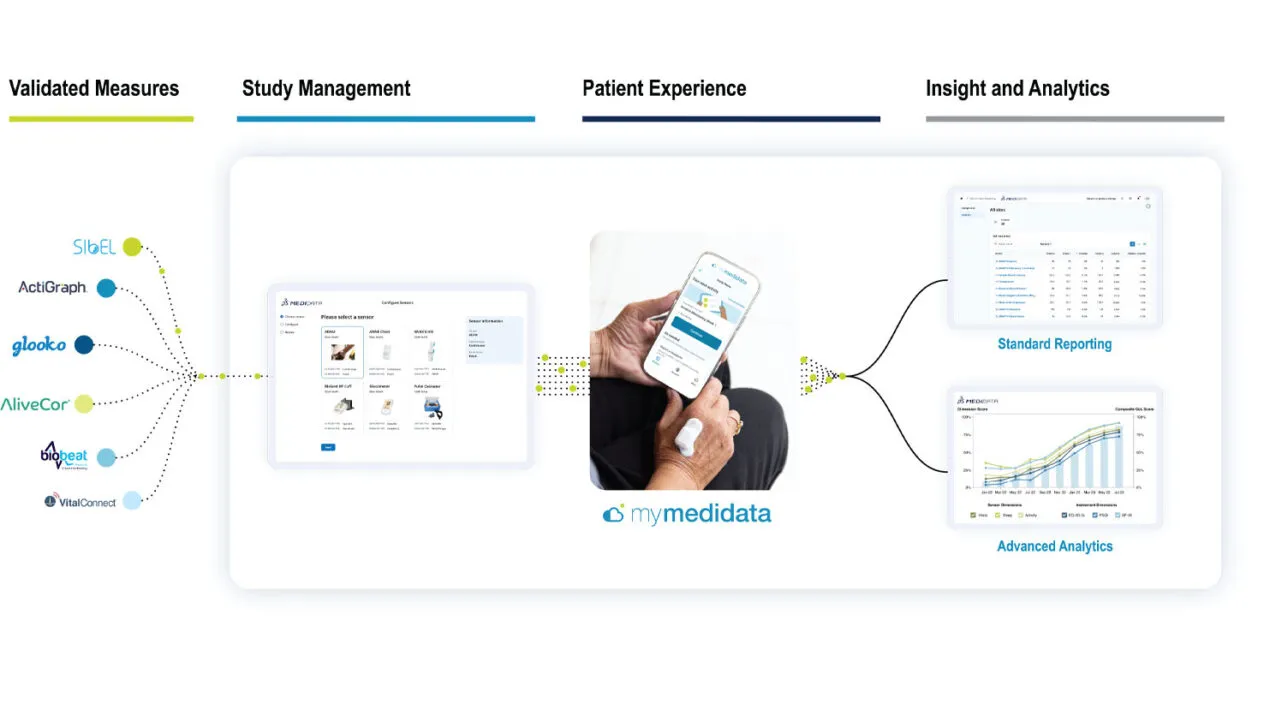
SensorCloud
A platform that integrates wearable sensor data with patient-reported outcomes, reducing burden while creating a fuller view of the trial experience.

Impact
The Patient Insights Program connected patient realities to roadmap decisions and gave our team a shared understanding of where trials feel confusing, rushed, or isolating. It created a practical way to validate choices from concept through release, and over time it shifted how we talked about success. Patient experience became part of our everyday conversations about tradeoffs and priorities, and collaboration across product, design, and engineering felt more human and more intentional, leaving the team with a clearer compass for future decisions.